Black mass primarily consists of the anode and cathode materials of batteries, which are rich in key metals such as lithium, cobalt, nickel, and manganese, generally comprising 40% to 60% of the total content.

Definition and Components of Black Mass
Black mass is a key component separated during the recycling process of lithium-ion batteries, primarily made up of the battery’s anode and cathode materials. During recycling, batteries are first disassembled and crushed, then physical and chemical methods are used to separate various materials, including black mass, which mainly contains metals like lithium, cobalt, nickel, and manganese, as well as carbon and binders used in the battery.
The metal content in black mass can reach 40% to 60%, with lithium content around 5% to 10%, and the content of cobalt, nickel, and manganese varies depending on the battery type. For instance, cobalt, as a rare metal, has a market price ranging from $30,000 to $50,000 per ton. Recycling cobalt can reduce the production costs of lithium-ion batteries by approximately 10% to 20%. From an environmental perspective, recycling these metals can reduce CO2 emissions by about 1.5 tons per ton.
A cost-benefit analysis of the recycling process shows that the treatment costs of black mass account for about 30% to 50% of the total recycling costs, but the savings on raw materials and environmental benefits far exceed the initial investment.
| Element | Percentage Content |
|---|---|
| Lithium | 5% – 10% |
| Cobalt | 10% – 20% |
| Nickel | 5% – 15% |
| Manganese | 5% – 10% |
| Others | 50% – 75% |
Role of Black Mass in Lithium-Ion Battery Recycling
Black mass plays a crucial role in the recycling of lithium-ion batteries, serving as a repository of metal resources within the battery. This material concentrates up to 40% to 60% of the metals, including but not limited to lithium, cobalt, nickel, and manganese, which are essential for manufacturing new batteries.
By recycling a ton of black mass containing cobalt, up to 80% of mining costs and the associated underground mining activities can be saved, thus reducing environmental damage and carbon emissions. The process of recovering materials like lithium and cobalt from black mass can effectively reduce the manufacturing costs of new batteries by an estimated 10% to 20%.
Black Mass Recycling Process
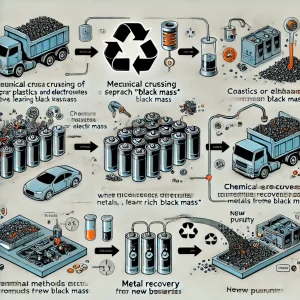
Batteries are first mechanically crushed, separating out plastics, electrolytes, and other non-metal materials, with the remaining part being rich in black mass. This process typically occurs in a sealed environment to prevent the leakage of hazardous chemicals.
Next, the crushed materials enter a chemical treatment phase, crucially using acid or alkaline solutions to dissolve valuable metals such as lithium, cobalt, nickel, and manganese from the black mass. The efficiency of this chemical process is about 90%, meaning nearly all recyclable metals can be extracted.
After metal extraction, the remaining solution is further processed through electrochemical methods or precipitation techniques to recover metals from the solution, efficiently retrieving cobalt and lithium with a purity of up to 99.5% through electrolysis. The operational costs of this step are about 30% of the entire recycling process, but the economic value of the recycled materials, which can be directly used in battery manufacturing, far exceeds the cost.
Finally, the purified metals are transformed into battery-grade compounds, such as lithium carbonate or lithium cobalt oxide, which are used in the production of new batteries. To better understand the entire process, below is a diagram of the recycling process: